CYBERDYNE Inc. of Japan are world leading innovative pioneers of AI-robotic solutions including the Hybrid Assistive Limb (HAL). Abdul Latif Jameel was the first to introduce HAL and ‘Cybernics Treatment’ into Saudi Arabia at Abdul Latif Jameel Rehabilitation Hospital, Jeddah, teaching Spinal Cord injury, stroke and other neuromuscular diseases patients to regain use of their limbs. Abdul Latif Jameel Health and CYBERDYNE have introduced a wholistic set of three different rehabilitation devices in Saudi Arabia Cyberdyne’s HAL- Lower Limb Type, HAL- Single Joint Type and the HAL- Lumbar Type.
Cyberdyne
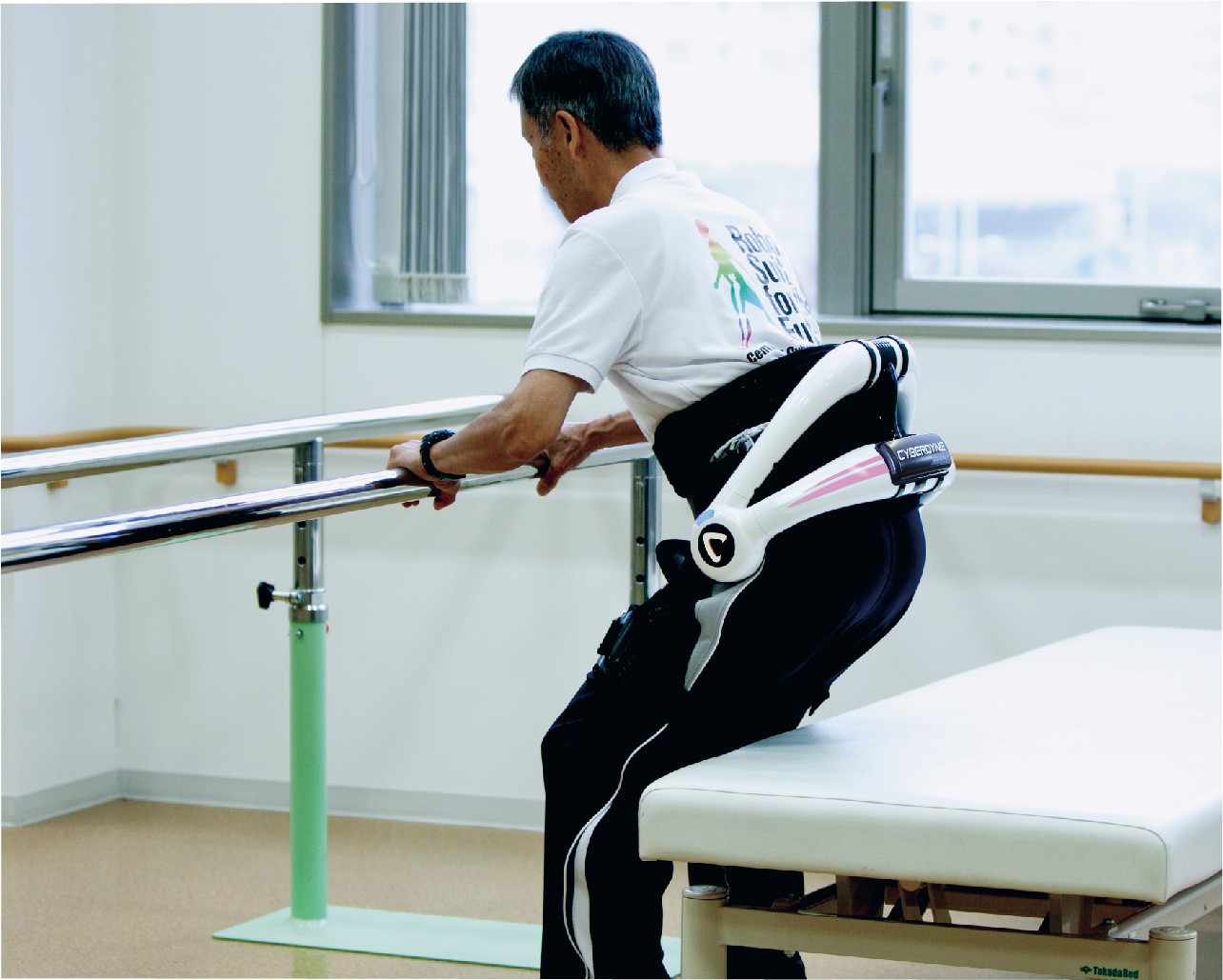
HAL for Medical Use Lower Limb Type
HAL for Medical Use – Lower Limb Type is a medical device for the rehabilitation of people with disorders in the lower limbs caused by diseases such as stroke, spinal cord injury, and eight types of neuromuscular diseases. By using the unique system of “HAL” to synchronize with the wearers’ intent and strengthen the connection of their nervous system through repeated interactive biofeedback, the product aims to improve and maintain the wearers’ ability to walk. Due to the accumulation of clinical evidence, including evidence that suggests the ability to delay the progress of neuromuscular diseases such as ALS, and even temporarily improve the users’ function, the device is approved and used in over 20 countries including US, EU, and Australia. This device has also received Saudi Food and Drug Authority’s authorization.

Publications :
- The Combined Efficacy of a Two-Year Period of Cybernic Treatment With a Wearable Cyborg Hybrid-Assistive Limb and Leuprorelin Therapy in a Patient With Spinal and Bulbar Muscular Atrophy: A Case Report
- Effects of Long-term Hybrid Assistive Limb Use on Gait in Patients with Amyotrophic Lateral Sclerosis
- Robot-assisted training using hybrid assistive limb ameliorates gait ability in patients with amyotrophic lateral sclerosis
- Frontiers | Treadmill Training with HAL Exoskeleton—A Novel Approach for Symptomatic Therapy in Patients with Limb-Girdle Muscular Dystrophy—Preliminary Study (frontiersin.org)
- Against the odds: what to expect in rehabilitation of chronic spinal cord injury with a neurologically controlled Hybrid Assistive Limb exoskeleton. A subgroup analysis of 55 patients according to age and lesion level – PubMed (nih.gov)
- Functional Neurorehabilitation using the Hybrid Assistive Limb (HAL): A First Experience in the United States | Request PDF (researchgate.net)
- A randomized controlled study incorporating an electromechanical gait machine, the Hybrid Assistive Limb, in gait training of patients with severe limitations in walking in the subacute phase after stroke | PLOS ONE
Certifications in major regions :
- U.S. FDA 510k Clearance.
- CE Marking [CE 0197].
- Japanese PMDA approval – authorization number 22700BZX00366000.
- Saudi SFDA approval – MDMA authorization number GHTF-2021-0763.
- ISO13485 (Medical Device).

HAL For Medical Use – Single Joint Type
HAL for Medical Use Single Joint Type is a product that is used for intensive rehabilitation of each joints by combining the main unit with the attachment suitable for various joints such as elbow, knee and ankle. Using the product, operator could perform rehabilitation in various positions, such as lying, sitting and standing in accordance to physical conditions of the patient. The compact design of the product makes it easy to carry around, enabling rehabilitation in a wide range of environments, such as general ward rehabilitation room and bedside room in the acute phases. This device has received its American FDA as Class I Medical device in 2021 and has received Saudi Food and Drug Authority in 2022.
Publications :
- Frontiers | Voluntary Elbow Extension-Flexion Using Single Joint Hybrid Assistive Limb (HAL) for Patients of Spastic Cerebral Palsy: Two Cases Report (frontiersin.org)
- Effect of Robot-assisted Rehabilitation to Botulinum Toxin A Injection for Upper Limb Disability in Patients with Chronic Stroke: A Case Series and Systematic Review
- Feasibility and efficacy of knee extension training using a single-joint hybrid assistive limb, versus conventional rehabilitation during the early postoperative period after total knee arthroplasty
Certifications:
- Listed in U.S. FDA as Class I Medical Device.
- EU CE Marking [CE 0197]
- Japanese PMDA approval – authorization number 302AIBZX00017000
- Saudi Food and Drug Authority MDMA authorization MDMA-2-2022-0740
- ISO 13485 (Medical Device).
HAL Lumbar type for Well-Being (Non-Medical)
HAL Lumbar Type for Well-being assists body trunk and lower limb training. The product comes with only 4 belts and 3 buttons, making it very easy to use. The battery powered and compactly designed device only weighs 3kg, allowing for its use in various situations such as group exercise at care facilities, or personal exercise in home care. If needed, this device can also be used by health care professionals such as physiotherapists, caregivers and nurses who undertake heavy lifting activities with exposure to musculoskeletal injuries just by switching the mode.
Certifications:
- EU CE Marking [Machinery Directive, 2006/42/EC]
- ISO 13482
For more Inquiries and Information related to our products